
LapBox®
MANUAL

Trusted by surgeons who demand efficacy,
safety, and simplicity in every procedure.

MANUAL
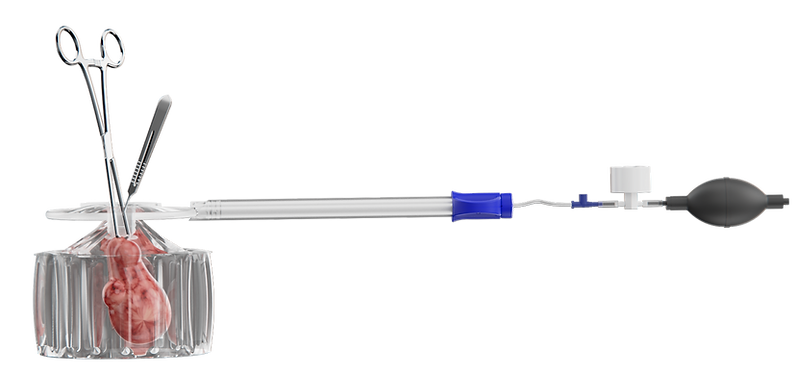
Engineered for manual morcellation and tissue removal, LapBox® Manual delivers dual-layer protection against spillage.
With its user-friendly, 'bag-in-bag' containment design, it integrates seamlessly into minimally invasive surgical workflows – providing enhanced control, continuous visualization, and safe, efficient tissue removal with a short learning curve.


DUAL
LAYER
EXTRA PROTECTION
EASY TO USE
DESIGN
SPILL
PROOF
SPILL-PROOF
FDA
CLEARED
SAFE SOLUTION

SMART & CONTROLLED
LapBox® Manual was purpose-built for precision in manual tissue extraction – offering secure, dual-layer containment with a design that feels intuitive from first use.
Surgeons benefit from enhanced control, clear visualization, and easy deployment, even in complex laparoscopic settings.
Off-Label Use Surgical Bags Rupture


FDA-Cleared Spill-Proof Double-Wall Containment System


PRECISION & PROTECTION
Optimized for minimally invasive gynecologic surgery, LapBox® Manual supports safe tissue removal with seamless workflow compatibility and a barrier you can trust.
By minimizing spillage risk during manual morcellation, it enables surgeons to protect patients – without compromising access or efficiency.


WHY CONTAINMENT MATTERS
In laparoscopic GYN surgery, the risks aren’t always visible.
Uncontained tissue fragments can seed serious complications, including the spread of undiagnosed cancer, endometriosis, or atypical fibroids.
Yet many current solutions leave surgeons choosing between safety and simplicity.
LapBox® bridges that gap — providing the protection patients deserve with the workflow surgeons need.

SILENT KILLER
1 in 350 women undergoing hysterectomy may have undiagnosed cancer
In a 2014 safety communication, FDA warned about the risk of spread of cancerous tissue within the abdomen and pelvis due to uncontained power tissue morcellation.
With few safe containment options available at the time, many surgeons were forced to shift toward open abdominal surgery, exposing patients to longer recovery times, greater pain, and higher complication rates.


FACTS & FIGURES
Hysterectomy is the second most frequently performed major gynecologic surgery in the U.S., with over 500,000 procedures each year.
1 in 350 women has an undiagnosed uterine cancer at time of hysterectomy. Countless others may have undiagnosed endometriosis, atypical fibroids, or precancerous tissue — all of which carry significant risk if unintentionally spread during surgery.








